The Cancer News
AN AUTHORITATIVE RESOURCE FOR EVERYTHING ABOUT CANCER
Research
The volume and pace of cancer research, from preclinical studies to clinical trials and FDA approvals, can make it challenging to stay informed. This section highlights the latest developments and translates complex scientific concepts into clear, accessible insights for both healthcare professionals and the broader public.
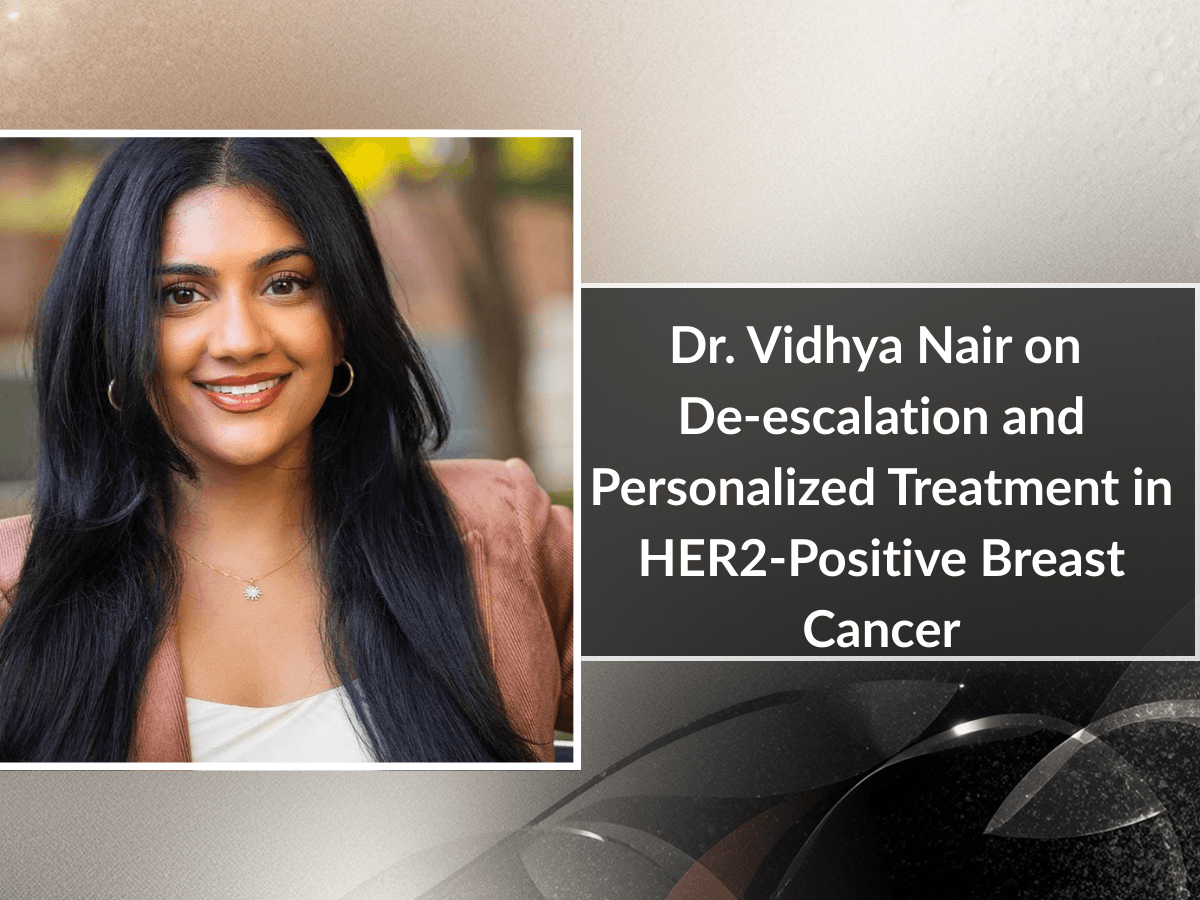
Dr. Vidhya Nair on De-escalation and Personalized Treatment in HER2-Positive Breast Cancer
By The Cancer News Team
Dr. Vidhya Nair reviews key advances in HER2-positive breast cancer, highlighting de-escalation in early-stage disease, T-DXd in high-risk and metastatic settings, and evolving personalized treatment strategies.

Dr. Vidhya Nair on De-escalation and Personalized Treatment in HER2-Positive Breast Cancer
By The Cancer News Team
Dr. Vidhya Nair reviews key advances in HER2-positive breast cancer, highlighting de-escalation in early-stage disease, T-DXd in high-risk and metastatic settings, and evolving personalized treatment strategies.

Dr. Vidhya Nair on De-escalation and Personalized Treatment in HER2-Positive Breast Cancer
By The Cancer News Team
Dr. Vidhya Nair reviews key advances in HER2-positive breast cancer, highlighting de-escalation in early-stage disease, T-DXd in high-risk and metastatic settings, and evolving personalized treatment strategies.
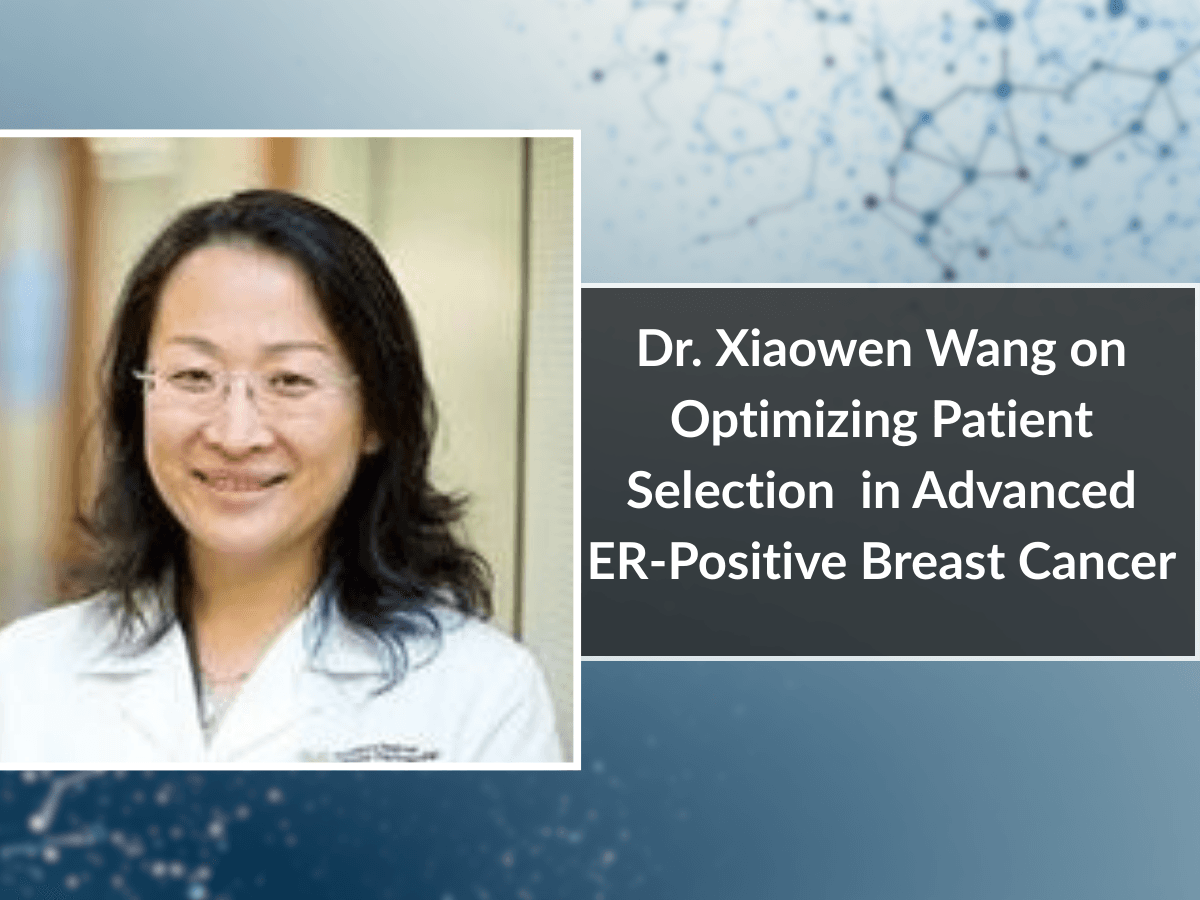
Dr. Xiaowen Wang on Optimizing Patient Selection in Advanced ER-Positive Breast Cancer
By The Cancer News Team
Dr. Xiaowen Wang reviews evolving strategies for advanced ER-positive metastatic breast cancer, highlighting NGS timing, ESR1 and PIK3CA mutations, novel SERDs, and targeted combination therapies.

Dr. Xiaowen Wang on Optimizing Patient Selection in Advanced ER-Positive Breast Cancer
By The Cancer News Team
Dr. Xiaowen Wang reviews evolving strategies for advanced ER-positive metastatic breast cancer, highlighting NGS timing, ESR1 and PIK3CA mutations, novel SERDs, and targeted combination therapies.

Dr. Xiaowen Wang on Optimizing Patient Selection in Advanced ER-Positive Breast Cancer
By The Cancer News Team
Dr. Xiaowen Wang reviews evolving strategies for advanced ER-positive metastatic breast cancer, highlighting NGS timing, ESR1 and PIK3CA mutations, novel SERDs, and targeted combination therapies.
Dr. Vivian G Oehler on Advances in Myeloproliferative Neoplasm Treatment
By The Cancer News Team
Dr. Vivian G. Oehler reviews major advances in myeloproliferative neoplasms, including pelabresib in myelofibrosis, CALR-targeted therapy in essential thrombocythemia, rusfertide in polycythemia vera, and new allosteric inhibitors in CML.
Dr. Vivian G Oehler on Advances in Myeloproliferative Neoplasm Treatment
By The Cancer News Team
Dr. Vivian G. Oehler reviews major advances in myeloproliferative neoplasms, including pelabresib in myelofibrosis, CALR-targeted therapy in essential thrombocythemia, rusfertide in polycythemia vera, and new allosteric inhibitors in CML.
Dr. Vivian G Oehler on Advances in Myeloproliferative Neoplasm Treatment
By The Cancer News Team
Dr. Vivian G. Oehler reviews major advances in myeloproliferative neoplasms, including pelabresib in myelofibrosis, CALR-targeted therapy in essential thrombocythemia, rusfertide in polycythemia vera, and new allosteric inhibitors in CML.
Dr. Ryan Cassaday on Recent Advances in the Treatment of Acute Lymphoblastic Leukemia
By The Cancer News Team
Dr. Ryan Cassaday reviews chemotherapy-free frontline strategies, MRD risk stratification, CAR T-cell consolidation, and transplant decision-making in adult acute lymphoblastic leukemia (ALL).

Dr. Ryan Cassaday on Recent Advances in the Treatment of Acute Lymphoblastic Leukemia
By The Cancer News Team
Dr. Ryan Cassaday reviews chemotherapy-free frontline strategies, MRD risk stratification, CAR T-cell consolidation, and transplant decision-making in adult acute lymphoblastic leukemia (ALL).

Dr. Ryan Cassaday on Recent Advances in the Treatment of Acute Lymphoblastic Leukemia
By The Cancer News Team
Dr. Ryan Cassaday reviews chemotherapy-free frontline strategies, MRD risk stratification, CAR T-cell consolidation, and transplant decision-making in adult acute lymphoblastic leukemia (ALL).

Dr. Mwanasha Hamuza Merrill on Frontline and Emerging Treatment Strategies for Chronic Lymphocytic Leukemia
By The Cancer News Team
At Best of Hematology & Breast Cancer 2026, Dr. Mwanasha Hamuza Merrill reviews frontline and emerging treatment strategies for chronic lymphocytic leukemia, highlighting CLL17, BRUIN 314, and novel BTK degrader data.

Dr. Mwanasha Hamuza Merrill on Frontline and Emerging Treatment Strategies for Chronic Lymphocytic Leukemia
By The Cancer News Team
At Best of Hematology & Breast Cancer 2026, Dr. Mwanasha Hamuza Merrill reviews frontline and emerging treatment strategies for chronic lymphocytic leukemia, highlighting CLL17, BRUIN 314, and novel BTK degrader data.

Dr. Mwanasha Hamuza Merrill on Frontline and Emerging Treatment Strategies for Chronic Lymphocytic Leukemia
By The Cancer News Team
At Best of Hematology & Breast Cancer 2026, Dr. Mwanasha Hamuza Merrill reviews frontline and emerging treatment strategies for chronic lymphocytic leukemia, highlighting CLL17, BRUIN 314, and novel BTK degrader data.
Dr. Ning Dong on Clinical Updates on Classification, Treatment, and Emerging Trials of T-Cell Lymphoma
By The Cancer News Team
Dr. Ning Dong reviews updated WHO classification, frontline and relapsed treatment strategies, prognostic modeling in advanced cutaneous T-cell lymphoma, and promising phase 2 trials including CD5 CAR T therapy.

Dr. Ning Dong on Clinical Updates on Classification, Treatment, and Emerging Trials of T-Cell Lymphoma
By The Cancer News Team
Dr. Ning Dong reviews updated WHO classification, frontline and relapsed treatment strategies, prognostic modeling in advanced cutaneous T-cell lymphoma, and promising phase 2 trials including CD5 CAR T therapy.

Dr. Ning Dong on Clinical Updates on Classification, Treatment, and Emerging Trials of T-Cell Lymphoma
By The Cancer News Team
Dr. Ning Dong reviews updated WHO classification, frontline and relapsed treatment strategies, prognostic modeling in advanced cutaneous T-cell lymphoma, and promising phase 2 trials including CD5 CAR T therapy.
Dr. Sioban B. Keel on Germline Cancer Predisposition in Myeloid Malignancies
By The Cancer News Team
Dr. Sioban B. Keel reviews germline cancer predisposition in myeloid malignancies, highlighting DDX41, donor selection, somatic mosaicism, and how inherited risk reshapes diagnosis, monitoring, and transplant decisions.

Dr. Sioban B. Keel on Germline Cancer Predisposition in Myeloid Malignancies
By The Cancer News Team
Dr. Sioban B. Keel reviews germline cancer predisposition in myeloid malignancies, highlighting DDX41, donor selection, somatic mosaicism, and how inherited risk reshapes diagnosis, monitoring, and transplant decisions.

Dr. Sioban B. Keel on Germline Cancer Predisposition in Myeloid Malignancies
By The Cancer News Team
Dr. Sioban B. Keel reviews germline cancer predisposition in myeloid malignancies, highlighting DDX41, donor selection, somatic mosaicism, and how inherited risk reshapes diagnosis, monitoring, and transplant decisions.

Dr. L. Christine Fang on Modern De-Escalation in Breast Cancer Radiation Therapy
By The Cancer News Team
Dr. L. Christine Fang reviews modern de-escalation strategies in breast cancer radiation therapy, including hypofractionation, partial breast irradiation, and radiation omission for selected patients.

Dr. L. Christine Fang on Modern De-Escalation in Breast Cancer Radiation Therapy
By The Cancer News Team
Dr. L. Christine Fang reviews modern de-escalation strategies in breast cancer radiation therapy, including hypofractionation, partial breast irradiation, and radiation omission for selected patients.

Dr. L. Christine Fang on Modern De-Escalation in Breast Cancer Radiation Therapy
By The Cancer News Team
Dr. L. Christine Fang reviews modern de-escalation strategies in breast cancer radiation therapy, including hypofractionation, partial breast irradiation, and radiation omission for selected patients.

Dr. Brie Chun on Who Really Needs More Adjuvant Therapy in Early-Stage Breast Cancer?
By The Cancer News Team
Dr. Brie Chun reviews long-term SOFT and TEXT trial data to clarify which premenopausal patients with early-stage hormone receptor–positive breast cancer benefit most from ovarian function suppression.

Dr. Brie Chun on Who Really Needs More Adjuvant Therapy in Early-Stage Breast Cancer?
By The Cancer News Team
Dr. Brie Chun reviews long-term SOFT and TEXT trial data to clarify which premenopausal patients with early-stage hormone receptor–positive breast cancer benefit most from ovarian function suppression.

Dr. Brie Chun on Who Really Needs More Adjuvant Therapy in Early-Stage Breast Cancer?
By The Cancer News Team
Dr. Brie Chun reviews long-term SOFT and TEXT trial data to clarify which premenopausal patients with early-stage hormone receptor–positive breast cancer benefit most from ovarian function suppression.
Dr. Emily N. Palmquist on De-escalation of Axillary Surgery in Early-Stage Breast Cancer
By The Cancer News Team
Dr. Emily N. Palmquist reviews evidence from SOUND, INSEMA, and BOOG trials on safely omitting sentinel lymph node biopsy in select patients with early-stage breast cancer.

Dr. Emily N. Palmquist on De-escalation of Axillary Surgery in Early-Stage Breast Cancer
By The Cancer News Team
Dr. Emily N. Palmquist reviews evidence from SOUND, INSEMA, and BOOG trials on safely omitting sentinel lymph node biopsy in select patients with early-stage breast cancer.

Dr. Emily N. Palmquist on De-escalation of Axillary Surgery in Early-Stage Breast Cancer
By The Cancer News Team
Dr. Emily N. Palmquist reviews evidence from SOUND, INSEMA, and BOOG trials on safely omitting sentinel lymph node biopsy in select patients with early-stage breast cancer.