The Cancer News
AN AUTHORITATIVE RESOURCE FOR EVERYTHING ABOUT CANCER
Bispecifics, CAR-T, and Advanced Therapeutics—Bridging the Access Divide

Professor of Medicine
CAR-T therapy and CD20/CD3 bispecific antibodies have transformed the treatment landscape for relapsed or refractory diffuse large B-cell lymphoma (DLBCL), offering new hope after chemotherapy failure. Yet for many patients, access to these advanced immunotherapies remains limited by geography, referral delays, insurance barriers, and socioeconomic challenges. In this perspective, Dr. Nandita Khera examines the widening access divide in lymphoma care and outlines practical, system-level solutions to ensure that innovation in cellular therapy and immunotherapy translates into equitable outcomes for all patients.
When James, a 62-year-old accountant, was diagnosed with diffuse large B-cell lymphoma (DLBCL), his oncologist reassured him that this was an aggressive disease, but often a curable one. Frontline chemoimmunotherapy was readily available at his community cancer center, and within weeks, James was in treatment, which put him into remission.
Then, the lymphoma came back after 6 months of finishing treatment. James’s referral to an NCI cancer center introduced him to the idea of chimeric antigen receptor T cell (CAR-T) therapy, now a standard of care for most patients with relapsed or refractory large B-cell lymphomas.
CAR-T represented hope and complexity, and timing is critical (Figure 1). Disease progression during manufacturing can render patients ineligible. Limited manufacturing slots, bed capacity, and staffing can extend timelines beyond what aggressive lymphoma biology allows.
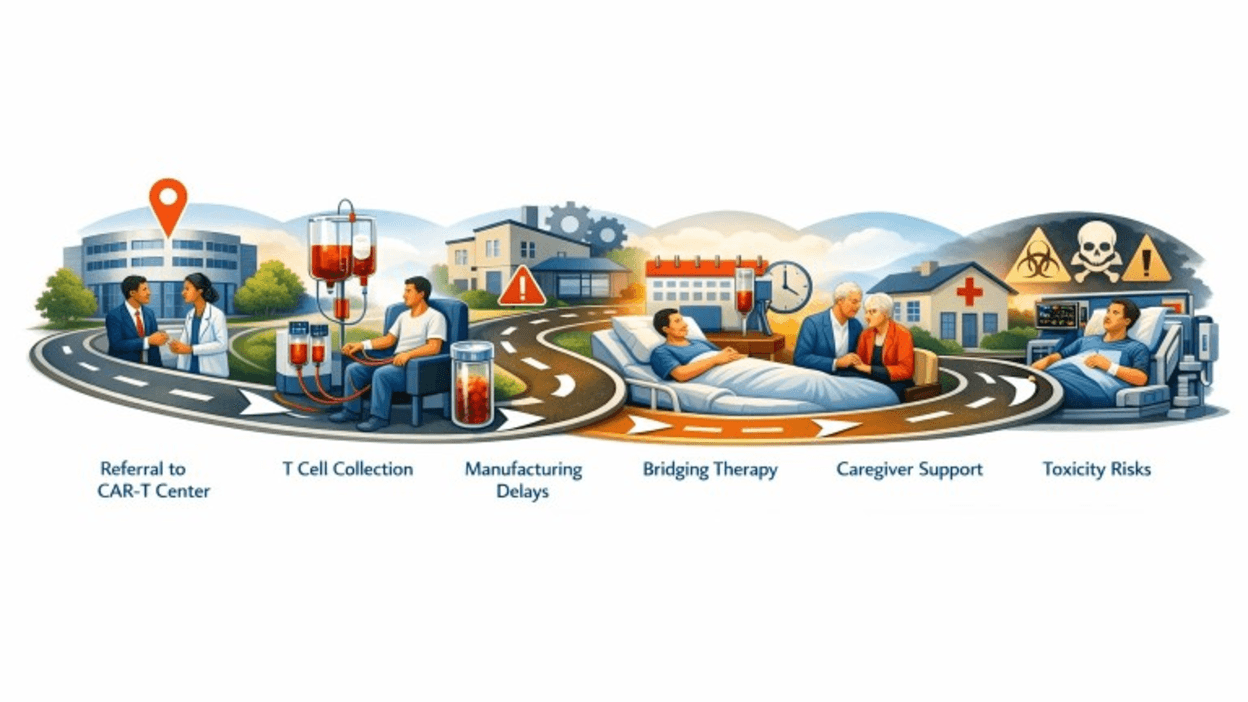
Figure 1: Complex CAR-T journey
James was fortunate to receive CAR-T through a coordinated model that should be standard, not exceptional. His local oncologist managed bridging therapy and labs. The CAR-T center managed lymphodepleting chemotherapy and CAR-T administration. His insurance case manager and a navigator at the CAR-T center helped with scheduling, insurance approvals, and caregiver education.
James responded initially, but the remission was incomplete. James’s oncologist discussed the next steps: CD20/CD3 bispecific antibodies, a rapidly expanding class of therapies reshaping lymphoma care. Unlike CAR-T, bispecifics are off-the-shelf, require no cell collection, and can often be started quickly, critical for patients with fast-moving disease. Early response rates in relapsed lymphoma are encouraging, even in patients who have exhausted multiple prior lines of therapy. For James, bispecific therapy offered immediacy. There was no manufacturing delay and no need to wait for a personalized product.
But access still came with caveats. Initial doses required extended outpatient monitoring away from home due to the risk of cytokine release syndrome and neurotoxicity. James lived two hours away. Each dose meant time off work, transportation planning, and reliance on his spouse for caregiving support. While insurance approved the drug, the hidden costs—travel, meals, lost income—accumulated quickly. His ability to get into complete remission made it worth it.
James was fortunate: He had good insurance, a motivated referring oncologist, strong caregiver support, and access to a high-volume center. Many patients are not. It is not surprising, therefore, that only about a third of patients who would benefit from these treatments are able to receive and benefit from them. (Figure 2)
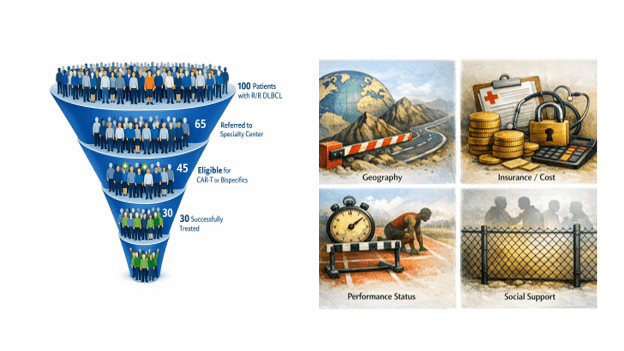
Figure 2: Access funnel with barriers for relapsed DLBCL
The Lymphoma Access Divide
Lymphoma care exposes access inequities with unusual clarity. Five barriers repeatedly surface in relapsed lymphoma:
- Geography: CAR-T and early bispecific treatments are concentrated in tertiary centers, often far from patients’ homes.
- Time: Referral and insurance delays collide with aggressive disease kinetics.
- Fitness and eligibility: Delays worsen performance status, making patients ineligible for intensive therapies.
- Socioeconomic strain: Caregiver requirements and travel disproportionately exclude vulnerable patients.
- Fragmented care: Lack of awareness of these treatments amongst community oncologists and poor coordination between community practices and specialty centers leads to lost time and lost opportunities.
These system barriers often augment challenges due to patient biology. The question no longer is: what is the standard of care? But what can this patient realistically get—and in time?
In relapsed or refractory lymphoma, speed matters. Disease biology can accelerate quickly, and delays in effective therapy translate directly into worse outcomes despite the availability of novel immunotherapies/ cellular therapies capable of inducing deep remissions after chemotherapy failure.
Redesigning lymphoma care delivery
Bispecific antibodies and CAR-T have fundamentally changed outcomes in relapsed lymphoma. For the first time, patients failing chemotherapy still have meaningful, potentially durable options. There is a need for scalable solutions to ensure that these advances are able to change the landscape for relapsed lymphoma:
- Early referral at first relapse: Do not wait for multiple recurrences before engaging advanced therapy centers.
- Hub-and-spoke lymphoma networks: Enable community oncologists to remain central, not sidelined.
- Standardized bridging protocols: Reduce variability and preserve eligibility.
- Virtual consultation and remote monitoring: Preserve access while minimizing burden.
- Resources for social support: Treat lodging and caregiver support as essential clinical infrastructure.
- Insurance Navigation and Advocacy: Proactive management of prior authorization and appeals and streamlined payer pathways.
- Equity metrics: Track access to CAR-T and bispecifics by race, language, distance, and payer status.
The Real Breakthrough in Lymphoma Care
Only about one-third of patients with refractory/relapsed DLBCL will have a happy conclusion like James'. One thing is clear: science is way ahead of the system. The real breakthrough in lymphoma will not come solely from a new target or construct. It will come from reengineering our care delivery processes and how patients move—from diagnosis to advanced therapy—without delay, duplication, or exclusion. This requires societal will and multi-stakeholder efforts to ensure that innovation in treatment matches innovation in access.
About Author
Nandita Khera is a Professor of Medicine at the Mayo Clinic College of Medicine and a Consultant in the Division of Hematology/ Oncology at Mayo Clinic Arizona. She treats patients with hematological malignancies and some solid tumors, especially those needing blood and marrow transplants (BMT) and/or cell therapy (CT). Her research interests include health economics, patient-reported outcomes, disparities in care, and dissemination/ implementation of research into practice in cancer. She has published several papers on outcomes and quality of care in cancer patients. She holds leadership positions on the various committees in organizations/ societies in hematology and BMT. She helps lead the patient-targeted efforts towards eliminating barriers to transplant and cell therapy as part of the ASTCT-National Marrow Donor Program's ACCESS Initiative. She is on the board of directors of ENGRAFT: a learning health network that strives to improve the lives of patients undergoing BMT/CT by bringing together all relevant stakeholders. She is the chair of the Therapeutic Strategy subcommittee at Mayo Clinic, where she works with a diverse team to bring transformative therapies to practice while ensuring financial stability for the institution. She teaches classes as part of the high value cost conscious care curriculum at Mayo Medical School and is a faculty member at large in the Science of Health Care Delivery (SHCD) Curriculum Subcommittee.
Works Discussed
- Battiwalla, M., Tees, M., Flinn, I., Pantin, J., Berdeja, J., Gregory, T., Maris, M., Bhushan, V., Vance, E., Mathews, J., Bachier, C., Shaughnessy, P., Ramakrishnan, A., Malik, S., Mori, S., Martin, C., Billups, R., Blunk, B., LeMaistre, C. F., & Majhail, N. S. (2025). Access barriers to anti-CD19+ CART therapy for NHL across a community transplant and cellular therapy network. Blood Advances, 9(2), 429-435. https://doi.org/10.1182/bloodadvances.2024014171
- Bock, A. M., & Epperla, N. (2025). Therapeutic landscape of primary refractory and relapsed diffuse large B-cell lymphoma: Recent advances and emerging therapies. Journal of Hematology & Oncology, 18(1), 68. https://doi.org/10.1186/s13045-025-01702-5
- Kansagra, A., Farnia, S., & Majhail, N. (2020). Expanding Access to Chimeric Antigen Receptor T-Cell Therapies: Challenges and Opportunities. American Society of Clinical Oncology Educational Book(40), e27-e34. https://doi.org/10.1200/EDBK_279151
- Shumilov, E., Scholz, J. K., Seib, M., Mazzeo, P., Wurm-Kuczera, R., Vucinic, V., Holtick, U., Boyadzhiev, H., Melchardt, T., Hölscher, A., Schultze-Florey, C., Abdelhafez, A., Velazquez, G. F., Ossami Saidy, A., Lesan, V., Schnetzke, U., Kerkhoff, A., Bacher, U., Ghandili, S., . . . Lenz, G. (2025). Outcomes of bispecific antibody therapy after CAR T-cell failure in relapsed/refractory large B-cell lymphoma. Blood Advances, 9(15), 3955-3966. https://doi.org/10.1182/bloodadvances.2024015719
- Westin, J., & Sehn, L. H. (2022). CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift? Blood, 139(18), 2737-2746. https://doi.org/10.1182/blood.2022015789