The Cancer News
AN AUTHORITATIVE RESOURCE FOR EVERYTHING ABOUT CANCER
Dr. Bart L Scott on Treatment Approaches in Newly Diagnosed Acute Myeloid Leukemia
Newly diagnosed acute myeloid leukemia (AML) presents complex treatment decisions that depend on patient fitness, disease risk, and emerging clinical trial data. At the Best of Hematology & Breast Cancer 2026 meeting in Seattle, Dr. Bart L. Scott of Fred Hutchinson Cancer Center reviewed contemporary first-line treatment strategies for AML, with a focus on azacitidine–venetoclax combinations, results from the Paradigm trial, and real-world clinical decision-making across diverse patient scenarios.
This article summarizes the presentation by Dr. Bart L Scott (Fred Hutchinson Cancer Center) at the Best of Hematology & Breast Cancer 2026, Seattle conference. It has not been reviewed by the speaker and may contain errors.
The session began with two clinical scenarios designed to frame the discussion around current treatment approaches for newly diagnosed acute myeloid leukemia (AML).
The first scenario involved a 77-year-old patient with newly diagnosed AML who presented with several comorbidities, including chronic kidney disease and heart failure, making them ineligible for standard intensive induction chemotherapy. The question posed was: Based on current data from landmark clinical trials, which of the following represents standard of care first-line therapy for this patient population? Options included high-dose cytarabine and mitoxantrone, azacitidine monotherapy, venetoclax along with azacitidine, or 7+3 chemotherapy.
The second scenario presented a 68-year-old male diagnosed with adverse risk FLT3 wild-type AML who was considered fit for intensive chemotherapy. Based on recent data from the Paradigm trial, attendees were asked to consider the most accurate rationale for the azacitidine combination over traditional cellular therapy. Options included: azacitidine-venetoclax demonstrated significant improvement in overall survival compared to 7+3 in transplant-eligible patients; 39% reduction in risk of progressive disease, relapse, or death (defined as event-free survival) with significantly shorter hospitalizations; intensive chemotherapy being contraindicated in all patients over age 65 regardless of fitness; or the combination being preferred because of lower rates of grade 3-4 [unclear].
NPM1-Positive IDH2-Positive AML with CNS Involvement: Case Management
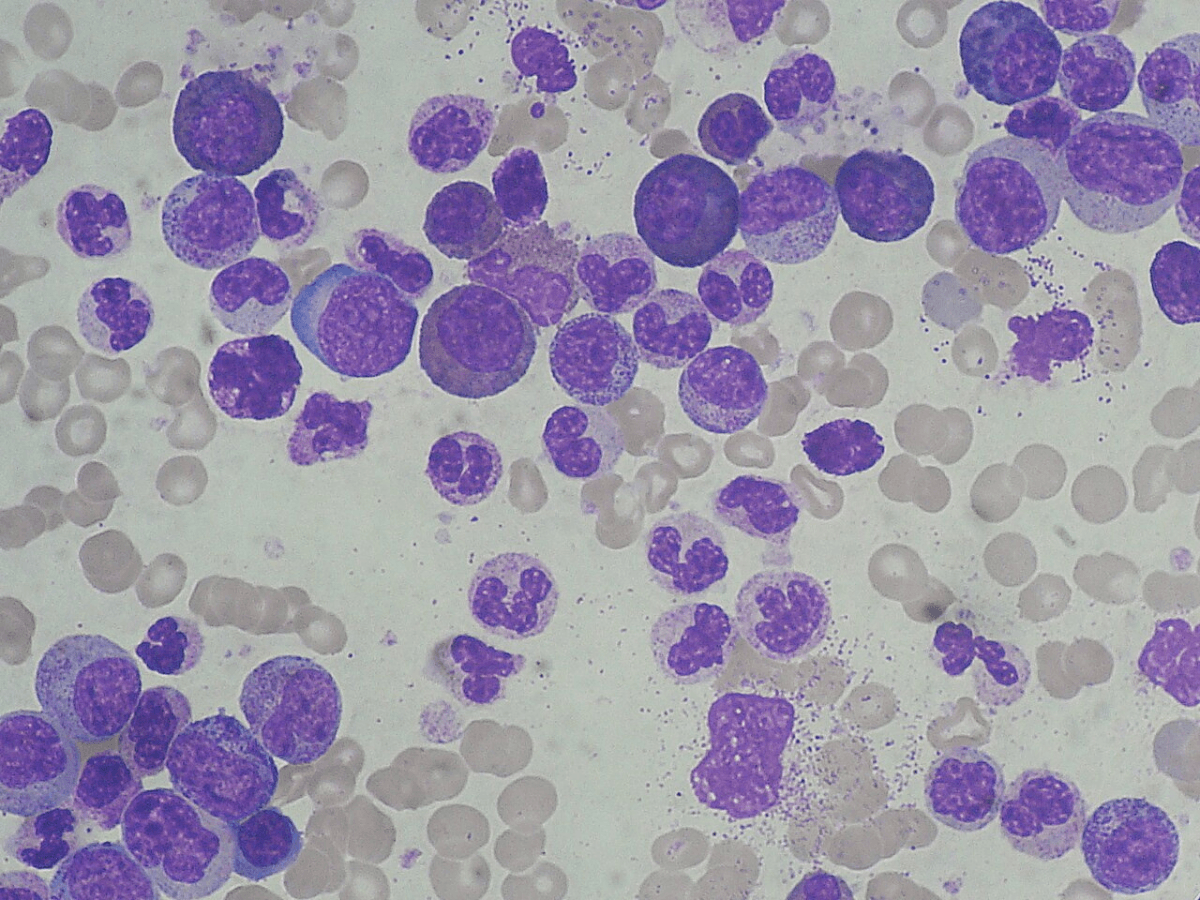
A detailed case was presented involving an 83-year-old male patient who initially presented with headaches and fever. The patient had multiple comorbidities, though not necessarily severe, including controlled hypertension, an ascending aortic aneurysm, benign prostatic hyperplasia on Flomax, and a history of recurrent deep vein thromboses and pulmonary embolisms on apixaban that was held due to low counts and thrombocytopenia. Notably, the patient had an incidental pituitary apoplexy with a 1.2-centimeter sellar mass, questionable pituitary macroadenoma.
On testing, the patient was NPM1-positive and IDH2-positive with an AML blast percentage of 52%. Cytogenetics showed CNS-positive disease based on cerebrospinal fluid testing, and the patient was classified as a favorable risk due to the NPM1 diagnosis. Rapid testing confirmed the initial diagnosis, and the patient did not have separate CEBPA mutations like the previously mentioned case.
Azacitidine-Venetoclax Treatment in AML: Initial Response and CNS Clearance
The patient received intrathecal chemotherapy initially, followed one week later by a second dose of weekly intrathecal therapy while laboratory testing was completed. The first cycle consisted of azacitidine-venetoclax at standard dosing, though due to azacitidine shortages, the dose was reduced as per practice patterns at the time. By the third intrathecal intervention, CNS flow cytometry was negative. The patient showed significant improvement both visually and in CNS confusion status.
Bone marrow assessment was performed not at day 14 as previously standard, but at day 21 or 24. This showed a favorable outcome based on next-generation sequencing with only 2% blasts remaining. The patient subsequently transitioned to outpatient care and received four additional intrathecal treatments. Approximately six weeks after induction, the patient received a second cycle of azacitidine-venetoclax.
Persistent Cytopenias After AML Induction: Monitoring and Management
A common challenge encountered with these patients is persistent, prolonged cytopenias. This prompted a bone marrow evaluation approximately two to three months into therapy. At this assessment, the patient remained in complete remission with less than 2% blasts, and NPM1 was negative, which was reassuring. Quantitative MRD analysis was also negative.
At this juncture, the clinical question became: what should be done next? The patient was experiencing cytopenias but was not transfusion-dependent, though counts remained low enough to cause significant functional impairment. The decision was made to continue with azacitidine and venetoclax. The patient did not develop differentiation syndrome and continued to do well.
AML Minimal Residual Disease Monitoring and Menin Inhibitor Considerations
The treatment plan included consideration for using menin inhibitors, which are newly approved drugs, in the event of progression. The monitoring strategy involved alternating next-generation sequencing testing between peripheral blood and bone marrow every four to six months.
Paradigm Trial in AML: Azacitidine-Venetoclax versus Intensive Chemotherapy
Discussion turned to data from the Paradigm study, which was presented as one of approximately 8,200 accepted abstracts at the conference. The study's presentation date was December 7th.
The primary endpoint chosen was event-free survival (EFS), which showed superiority for the azacitidine-venetoclax combination. The rationale for selecting EFS as the primary endpoint, rather than overall survival, was that it serves as a surrogate for overall survival. This decision was influenced by the high rate of crossovers among patients who progressed and changed therapies, making overall survival less reliable as an endpoint.
Event-Free Survival and Complete Remission Rates in AML
Event-free survival favored the azacitidine-venetoclax arm at 14.6 months versus 6 months for intensive chemotherapy. The overall response rate was significantly better with the combination therapy. Composite remission rates showed improvement with the combination, though the complete remission rates were similar between arms. This similarity in CR rates despite better statistical outcomes in other measures was noted as particularly interesting and important for answering the pre-test questions.
AML Treatment-Related Mortality and Hospitalization Outcomes
The 60-day mortality rate favored the azacitidine-venetoclax combination at 2.7% compared to intensive chemotherapy. ICU admission rates were also lower with the combination approach. Perhaps most notably, median hospitalizations were dramatically reduced with the combination therapy: only 15 days compared to 36 inpatient days with intensive chemotherapy. This difference was emphasized as phenomenal, given the importance of prioritizing quality of life over quantity of life for patients.
AML First-Line Therapy Selection in Unfit Patients: Evidence-Based Decision-Making
Returning to the first clinical scenario of the 77-year-old patient with newly diagnosed AML, multiple comorbidities, and unfitness for intensive induction chemotherapy, the question was posed again: based on current guidelines and landmark trials conducted before recent data from Verona and other studies from the past two months, what treatment approach would be appropriate?
When asked who would choose high-dose chemotherapy with mitoxantrone, the consensus was negative. The choice then became whether to use azacitidine as a single agent or in combination with venetoclax. Based on recent data, the combination approach was supported. This represents a patient population that would have been enrolled in the randomized multicenter controlled trial showing better outcomes. The combination is generally less sensitive to persistent cytopenias compared to other approaches.
TP53-Mutated AML and Patient Fitness Considerations
A critical caveat was raised regarding TP53-mutated patients. When examining the subset of patients with TP53 mutations, there was no benefit of combination therapy for these specific patients, making this an important exclusion to consider.
Age was also discussed as an important consideration, though the emphasis was placed on physiologic fitness rather than chronologic age. The question is not simply the patient's age, but whether they are physiologically fit.
FLT3 Wild-Type AML Treatment: Paradigm Study Clinical Applications
For the second clinical scenario involving newly diagnosed FLT3 wild-type AML in a functionally fit patient, the question focused on the most accurate rationale for choosing azacitidine-venetoclax based on current data. The correct answer was identified as option B: 39% reduction in risk of progressive disease, relapse, or death (event-free survival) with significantly shorter hospitalizations.
AML Clinical Trial Design: Event-Free Survival versus Overall Survival Endpoints
Important questions were raised about whether a phase three study is needed or whether the current evidence is sufficient to change practice. Discussion centered on the most challenging piece of information: whether the benefit in event-free survival is driven by early assessment during induction therapy, which might invalidate the endpoint. Notably, there was no difference in overall survival between treatment arms.
Several limitations of the trial were highlighted: EFS as an endpoint is problematic, overall survival is likely influenced by the high crossover rate, and there was no significant difference in overall survival with reasonable follow-up. The 60-day mortality findings were also noted as potentially problematic given the crossover issues.
Changing AML Treatment Paradigm: Shift from Intensive Chemotherapy to Lower-Intensity Regimens
Despite these limitations, there was consensus that the study would change the landscape of therapy. The expectation is that more patients will be treated with azacitidine-venetoclax, potentially resulting in fewer patients being referred to clinical trials and more patients being treated locally with this combination. While this shift is not necessarily viewed as entirely positive, it was acknowledged as the reality of the situation.
The Paradigm trial will likely be considered a positive clinical trial because it met its primary endpoint of superior EFS, and this will be marketed accordingly as a successful study.
Favorable-Risk AML: Preserving Intensive Chemotherapy for Curable Disease
A critical reminder was emphasized: favorable-risk AML patients were generally excluded from the Paradigm trial, as these patients are best served by intensive chemotherapy. There was strong consensus not to stop referring favorable-risk patients for intensive treatment, as these patients can be cured with chemotherapy. The movement toward less intensive therapy should not be applied indiscriminately to all AML patients, particularly those with favorable-risk disease who benefit from curative intensive approaches.